TRYPSIN SE (PANCREATIN) is a protease complex comprising the core enzymes produced by the pancreas. This endows TRYPSIN SE (PANCREATIN) with broad enzymatic activities, including trypsin, chymotrypsin, lipase, elastase, amylase, and carboxypeptidase B (with ECs 3.4.21.4, 3.4.21.1, 3.1.1.3, 3.4.21.36, 3.2.1.1, 3.4.17.2). This enzymatic profile allows faithful mimicking of the normal digestive functions of the pancreas, making Trypsin SE particularly well suited to applications requiring the digestion of fats, carbohydrates, and proteins.
Applications
Infant Formula; Digestibility and Hypoallergenic solutions
The cows’ milk source of most commercially available infant and follow-on formula makes babies at high risk of milk allergy susceptible to serious adverse reactions. TRYPSIN SE (PANCREATIN) can be used to produce hypoallergenic infant formula by hydrolysing milk proteins into smaller, non-allergenic polypeptides. This pre-digestion can also be used to aid the digestibility of infant formulae, making the constituent polypeptides and amino acids more readily bioavailable.
Whey Protein Hydrolysis
TRYPSIN SE (PANCREATIN) is used for the hydrolysis of whey protein. Hydrolysis of whey improves amino acid bioavailability, promoting rapid delivery into the bloodstream for muscle repair and recovery after intensive exercise. This hydrolysis process also renders hydrolysed whey protein more readily digestible for those with lactose intolerance.
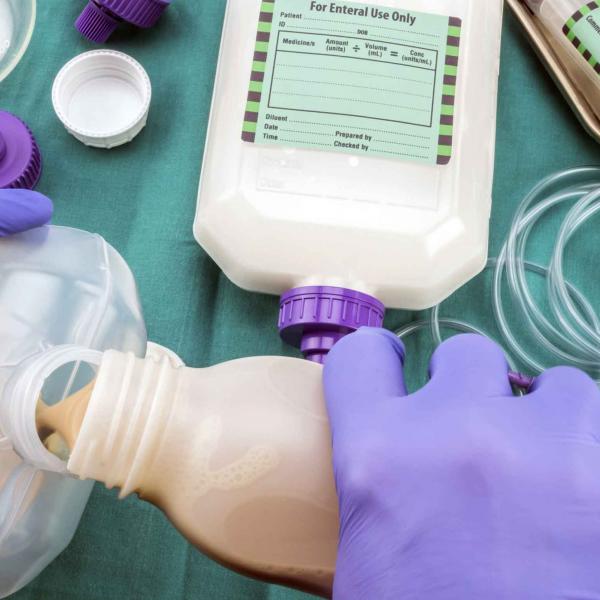
Medical food and enteral feeding
Digestive disorders arising from pancreatic insufficiency require pancreatic enzyme replacement therapy (PERT) to avoid the serious risk of malnutrition. TRYPSIN SE (PANCREATIN) can be used in the formulation of therapies that replace pancreatic enzymes, aiding patients’ digestion of proteins, starches, and lipids.
Specifications (USP units/mg):
Activity in USP units/mg
Protease: ≥ 250 U/mg
Regulatory
- EFSA (European Food Safety Authority) declared no safety concern for use as food enzyme (EFSA Journal Vol. 19, No.1 Jan 2021)
- Expected to be included on European Commission list of food enzymes in 2023
Trypsin Grades
Medical, Pharmaceutical, and Research
| Application Suitability | TRYPSIN VI | TRYPSIN IV | TRYPSIN II | TRYPSIN I | TRYPSIN 1:250 | TRYPSIN SE Pancreatin |
|---|---|---|---|---|---|---|
| Vaccine Production | ||||||
| Cell and Tissue Culture | ||||||
| Mass Spectroscopy | ||||||
| Diabetes & Ophthalmic research | ||||||
| USP / EP guideline conformance | ||||||
| Reduce Oedema and Bruising | ||||||
| Enteral Feeding |
Food Processing, Infant Formula & Dietary Supplements
| Application Suitability | TRYPSIN VI | TRYPSIN IV | TRYPSIN II | TRYPSIN I | TRYPSIN 1:250 | TRYPSIN SE Pancreatin |
|---|---|---|---|---|---|---|
| Whey Protein Hydrolysis | ||||||
| Production of Infant Formula | ||||||
| Production of Hypoallergenic Infant Formula | ||||||
| Food Processing | ||||||
| Meat and Seafood Tenderisation | ||||||
| Enhancement of Food Digestibility | ||||||
| Dietary Supplementation | ||||||
| Functional Foods |
Neova Technologies Inc., part of Bioseutica® group, has been extracting pancreatic enzymes for over 25 years. As one of the largest global manufacturers of porcine-derived enzymes, we have the experience to tailor our preparations to the exacting specifications of our customers. Contact us using the form below to discuss your specific requirements.
No Warranty: The information contained herein is provided in good faith and, to the best of our knowledge, is true and correct. However, no warranty or guarantee is implied or inferred and the information may be subject to change without prior notice. Neova Technologies Inc.’s products are sold with the understanding that the purchaser will conduct tests to determine the suitability of these products for their particular use
Bioseutica® is proud to inform that the European Food Safety Authority (EFSA) has just issued its scientific opinions on two applications submitted by NEOVA Technologies Inc (a BIOSEUTICA group company) related to the food enzymes
- trypsin and chymotrypsin from porcine pancreas (our Brand name TRYPSIN VI)
- trypsin, chymotrypsin, elastase and carboxypeptidase from porcine pancreas (our Brand name TRYPSIN SE)
The EFSA conclusions for the 2 enzymes are:
“Based on the origin of the food enzyme from edible parts of animals, the data provided by the applicant, supported by the evaluation of clinical studies with pharmaceutical preparations based on pancreatic enzymes, the Panel concluded that the porcine pancreatic enzymes do not give rise to safety concern under the intended conditions of use.”
Access to the 2 scientific opinions published on the EFSA Journal Vol. 19, No. 1, January 2021 is freely available here.
Based on these EFSA safety evaluations, the 2 food enzymes will be approved by the European Commission by means of their inclusion into the Union list of food enzymes, expected in 2023.
These positive opinions are a confirmation of the commitment the Bioseutica group has to the quality and safety of its products, as well as to our dedication to service to our customers and end-users.